Understanding the structure of gold and platinum nanoclusters
The Challenge:
Gold and platinum nanoclusters have unique properties that can be used in many industries, but because of their size they are difficult to model.The Solution:
To use NeSI’s Mahuika supercomputer to calculate potential low-energy nanoclusters and compare them to experimental work.The Outcome:
An accurate computational protocol for calculating the structures of industrially-relevant nanoclusters that can be applied to a range of metals and inform future research.
Nanoclusters are particles around one million times smaller than a centimetre in size, made of dozens of atoms. At this size, these particles have interesting chemical and physical properties that make them useful in medicine, clean energy and chemical processing.
Dr Anna Garden is a computational chemist at University of Otago. By using NeSI supercomputers, Anna and her team were able to calculate the energies of different nanoparticle structures of gold and platinum atoms.
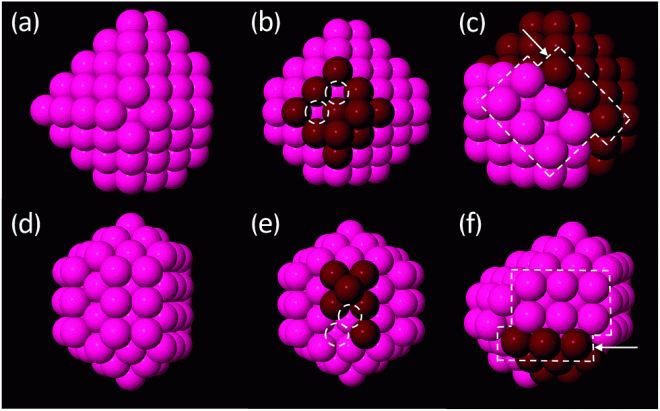
The nanoclusters Anna and her team studied had been synthesised through laboratory experiments in the past. These clusters were between 55 and 309 atoms in size with the potential to be used as catalysts in chemical reactions, including hydrogen fuel storage.
“Our collaborators in the UK discovered gold and platinum nanoparticles have quite different structures, which is surprising because they’re neighbours on the periodic table; you’d assume they’d behave similarly. We therefore wanted to performed calculations to figure out the possible shapes these particles could form and hopefully lend some insight into why the two metals had different structures,” Anna said.
Two different methods were used to discover these shapes. The first was a series of calculations using a global optimisation algorithm. This allowed the team to find which structures had the lowest energy – representing the most stable structures of the nanoclusters and therefore likely shapes to be observed experimentally.
“The global optimisation involved very many small calculations. These calculations don’t use many CPU hours and don’t have high memory requirements, but they needed to be run in parallel. These calculations also create a large number of files; NeSI was able to store these files and help us navigate them.”
The second part of the project was to calculate more accurate energies for these optimised structures. For this, Anna’s team used quantum chemical software to accurately model the physics of these nanoclusters.
In computational chemistry, the physics of heavy atoms like platinum and gold are much more difficult to model than light atoms like hydrogen due to the very large number of electrons. To understand the behaviour of nanoclusters formed by these heavy atoms, Anna’s team required a large amount of computational power. This is where NeSI’s Mahuika supercomputer became vital to the project.
“These were jobs that required multiple cores and nodes to calculate. We didn’t want to just pick a random sample of structures to look at, because we may have picked anomalies. Instead, we tested different brackets of nanocluster sizes. This burned through a lot of CPU time but added significant value to the project.”
Using these two types of calculations, Anna’s team were able to recreate the structures created through experiment and explain their behaviour. This also created a reference for accurately simulating other nanoclusters in the future. With this groundwork, the team can rely on the same calculations to discover optimised new structures. The graduate students who worked on this project, Stephanie Lambie and Geoffrey Weal are now continuing this work with NeSI, applying the calculations to other metals, including copper clusters.
“Working with NeSI was very smooth. We were able to do these two very different types of calculation without significant down-time, despite having new people come onboard halfway through. The students communicated with NeSI staff who got them set up, deal with issues, and inform them when we filled our quota,” Anna said.
NeSI’s support of University of Otago researchers like Anna allows this kind of foundational work to exist and continue, leading to international collaboration, new chemical properties discovered for industry, and new research opportunities.
Do you have an example of how NeSI platforms have supported your work? We’re always looking for projects to feature as a case study. Get in touch by emailing support@nesi.org.nz.