Quasi-2d Gallium Nanomaterials
Alongside silicon, gallium plays a major role in our modern technological world as a semiconductor component. It is a unique metal with many interesting properties, including how it melts.
Normal metals, such as alumunium or silver, melt at very high temperatures (650–950◦ C). A chunk of gallium metal, however, will melt in the human hand, needing less than body heat (37◦ C) to liquefy. At the nanoscale the intrigue continues: compared to larger chunks, smaller bits of matter typically melt at lower temperatures owing to a greater percentage of surface area which is more susceptible to temperature. However, a minuscule amount of gallium (tens of atoms, deemed a “nanocluster") remains solid at more than 250◦ C (the highest oven setting). The origin of this strange “greater-than-bulk" melting phenomenon has long remained a mystery, making it difficult to technologically exploit.
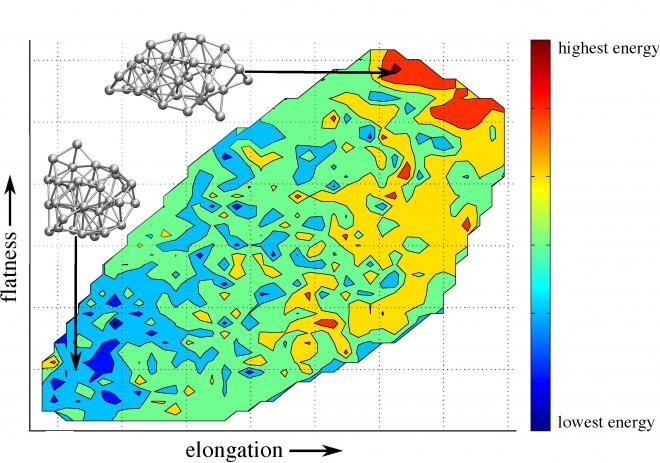
Dr Krista G. Steenbergen (Massey University, Albany) and Dr Nicola Gaston (Victoria University of Wellington) have recently unlocked the secret to greater- than-bulk melting of gallium nanoclusters. [1] Through an extensive series of computational simulations and analysis on NeSI’s Pan and Foster systems, they discovered a counterintuitive quasi-two-dimensional (quasi-2D) liquid structure in nanoscale gallium. At the nanoscale, typical liquids adopt a spherical geometry as this geometry minimises the surface area. In gallium however, liquid nanoclusters prefer a flattened-elongated, quasi-2D structure that actually maximises the surface area. Intriguingly, these quasi-2D liquid structures are stable despite a strong energetic preference for spherical shapes (see illustration).
To explain this anomaly, the researchers compared these small clusters to the highly stable surface structure of bulk gallium. It has long been known that the gallium bulk surface is more metallic (better at conducting) than the interior structure of gallium – an increased metallic character is also observed for the quasi-2D liquid nanostructures. Further investigation revealed that the flattened nanostructures are, in fact, structural approximates of the bulk gallium surface bilayer. The structural preference for this particular quasi-2D geometry places constraints on the normally unconstrained liquid state. In physics terms, these constraints lower the entropy of the nanoliquid state which, in turn, raises the melting temperatures of gallium nanoclusters.
On a practical level, the stability of the gallium surface structure has already found application in the growth of gallium nitride (GaN) films - where the stable gallium surface bilayer controls the kinetics of the film growth and results in improved morphology. GaN is a powerful semiconductor material used for high-performance transistors and integrated circuits. At a fundamental level, these findings solve a ten year-old mystery, and reveal the importance of both shape and dimensionality in the melting of nanoscale systems.
Simulations
In order to complete this work, Dr Gaston and Dr Steenbergen made extensive use of both NeSI’s Pan (University of Auckland) and Foster (University of Canterbury) high performance computing (HPC) clusters. The calculations were performed using VASP at the first-principles level of theory (density functional theory, DFT), requiring billions of highly-parallel calculations that considered both ionic and electronic degrees of freedom. The simulations included 13 gallium nanocluster sizes ranging from 7-36 atoms. The most recent simulations calculated the melting temperature of bulk gallium at the first-principles level of theory. Very few DFT bulk melting simulations have been completed to date, due to the enormous computational scale of the problem. The calculation requires a large periodic supercell (containing 200-500 atoms), which has only recently become possible at the first-principles level of theory with the advent of increasingly-parallel computational algorithms and access to HPC resources, such as those offered by NeSI. “We are very grateful to NeSI – both for the access to powerful HPC resources as well the excellent computational support provided by Gene Soudlenkov, Francois Bissey, Jordi Blasco, Ben Roberts and the rest of the NeSI team."
Krista G. Steenbergen and Nicola Gaston: kgsteen@gmail.com; nicola.gaston@vuw.ac.nz
[1] Krista G. Steenbergen and Nicola Gaston. “A 2D liquid structure explains the elevated melting temperatures of gallium nanoclusters," Nano Letters, DOI: 10.1021/acs.nanolett.5b02158 (2015).
Figure 1: An example potential energy surface for a liquid gallium nanocluster (36-atoms). The lower-left corner of the plot represents the energy for spherically- shaped nanoclusters; the upper right corner represents the energy for the flattened- elongated structures; blue corresponds to the lowest energy (energetically preferred), while red is high energy. Clearly, the flattened-elongated nanoclusters have the highest energy, yet they are counterintuitively stable within the liquid phase.